Prioritize the substituents at each chiral center and then, by comparing them to the models you created in Section 6.3.2, label the absolute configuration as R or S.
(d)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: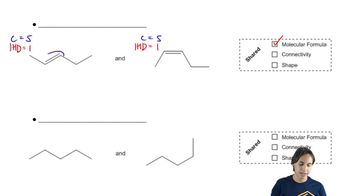


 1:48m
1:48mMaster Why stereoisomers need their own naming system. with a bite sized video explanation from Johnny
Start learning