Draw all the isomers that have molecular formula C5H11Br. (Hint: There are eight.)
a. Give the systematic name for each of the isomers.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: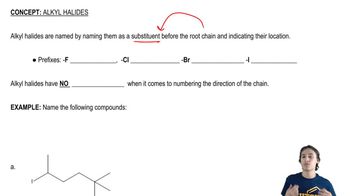

 1:52m
1:52mMaster How to name alkyl halides with a bite sized video explanation from Johnny
Start learning