Draw a constitutional isomer for C4H11N containing a 1° amine at a
(a) 1° carbon,
(b) 2° carbon, and
(c) 3° carbon.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
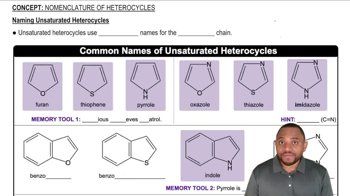

 1:52m
1:52mMaster How to name alkyl halides with a bite sized video explanation from Johnny
Start learning