Textbook Question
Name the following alkanes using the IUPAC system of nomenclature. [Each molecule exemplifies one of the nomenclature rules in Tables 3.7 and 3.8.]
(k) rule 6
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: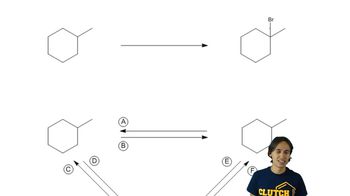

 3:43m
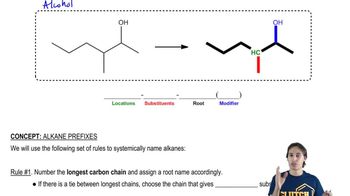
3:43mMaster The different parts of an IUPAC name with a bite sized video explanation from Johnny
Start learning