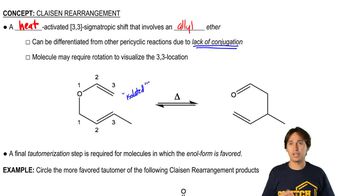
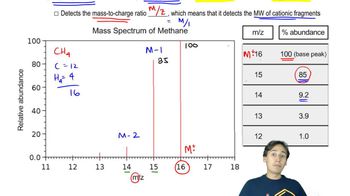
The ultimate test of fluency in MS and IR is whether you can determine a moderately complex structure from just the MS and the IR, with no additional information. The IR and MS of a compound are shown below. Use everything you know about IR and MS, plus reasoning and intuition, to determine a likely structure. Then show how your proposed structure is consistent with these spectra.
<IMAGES>