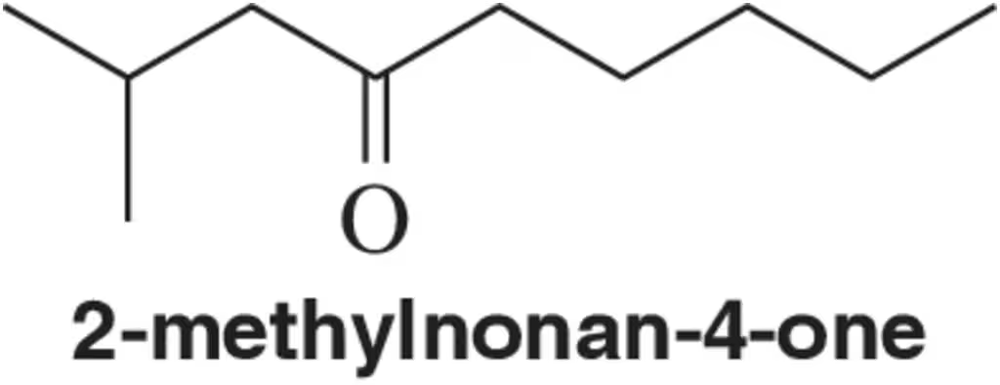
A student added 3-phenylpropanoic acid (PhCH2CH2COOH) to a molten salt consisting of a 1:1 mixture of NaCl and AlCl3 maintained at 170 °C. After 5 minutes, he poured the molten mixture into water and extracted it into dichloromethane. Evaporation of the dichloromethane gave a 96% yield of the product whose spectra follow. The mass spectrum of the product shows a molecular ion at m/z 132. What is the product?
<IMAGE>