An unknown compound gives the following mass, IR, and NMR spectra. Propose a structure, and show how it is consistent with the spectra. Show the fragmentations that give the prominent peaks at m/z 127 and 155 in the mass spectrum.
<IMAGES>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: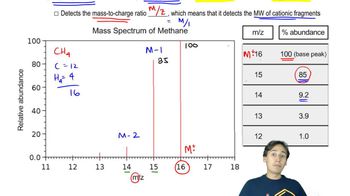

 4:28m
4:28mMaster Ionization Potentials with a bite sized video explanation from Johnny
Start learning