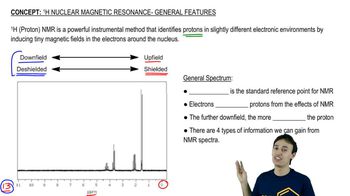
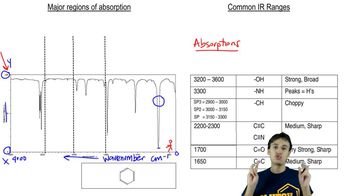
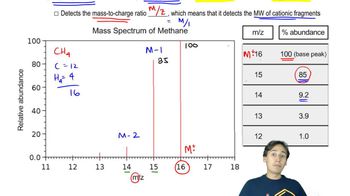
An acid-catalyzed reaction was carried out using methyl cellosolve (2-methoxyethanol) as the solvent. When the 2-methoxyethanol was redistilled, a higher-boiling fraction (bp 162°C) was also recovered. The mass spectrum of this fraction showed the molecular weight to be 134. The IR and NMR spectra are shown here. Determine the structure of this compound, and propose a mechanism for its formation.
<IMAGE>