Write structural formulas for the following compounds (includes both old- and new-style names).
(a) 2-octyne
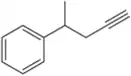
(b) ethylisopentylacetylene
(c) ethynylbenzene
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:55m
1:55mMaster How to name alkenes and alkynes with a bite sized video explanation from Johnny
Start learning