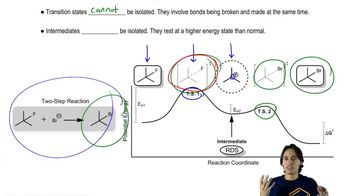
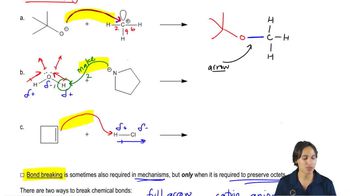
For the following equilibrium processes and the corresponding ∆G°, calculate (i) Keq and (ii) the % composition of the equilibrium mixture (% reactants, % products) at 298 K.
(b)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 5:02m
5:02mMaster Breaking down the different terms of the Gibbs Free Energy equation. with a bite sized video explanation from Johnny
Start learning