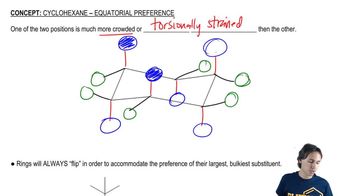
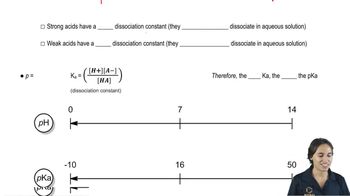
For the following acid–base reactions studied in Assessment 5.25, draw a likely transition state. Be sure to indicate in your drawing the degree to which bonds are broken or formed.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: