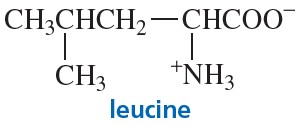
For many centuries, the Chinese have used extracts from a group of herbs known as ephedra to treat asthma. A compound named ephedrine has been isolated from these herbs and found to be a potent dilator of air passages in the lungs.
a. How many stereoisomers does ephedrine have?
b. The stereoisomer shown here is the one that is pharmacologically active. What is the configuration of each of the asymmetric centers?