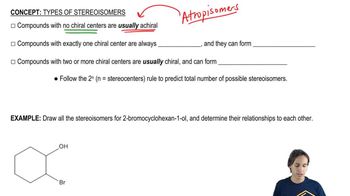
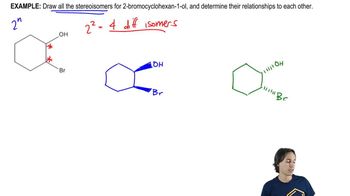
Draw a three-dimensional structure for each compound, and star all asymmetric carbon atoms. Draw the mirror for each structure, and state whether you have drawn a pair of enantiomers or just the same molecule twice. Build molecular models of any of these examples that seem difficult to you
(a)
(b)