Draw the stereoisomers of the following amino acids. Indicate pairs of enantiomers and pairs of diastereomers.
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: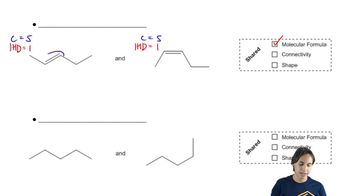

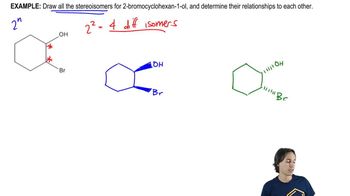

 3:51m
3:51mMaster Determining when molecules are different. with a bite sized video explanation from Johnny
Start learning