Textbook Question
The triphenylmethyl cation is so stable that some of its salts can be stored for months. Explain why this cation is so stable.
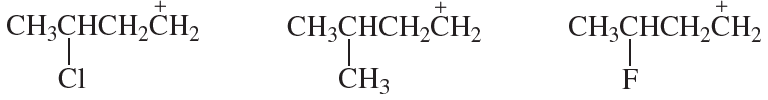
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 5:58m
5:58mMaster Determining Carbocation Stability with a bite sized video explanation from Johnny
Start learning