Textbook Question
Rank the following carbocations in order of stability (1 = most stable; 5 = least stable ). Explain your order.
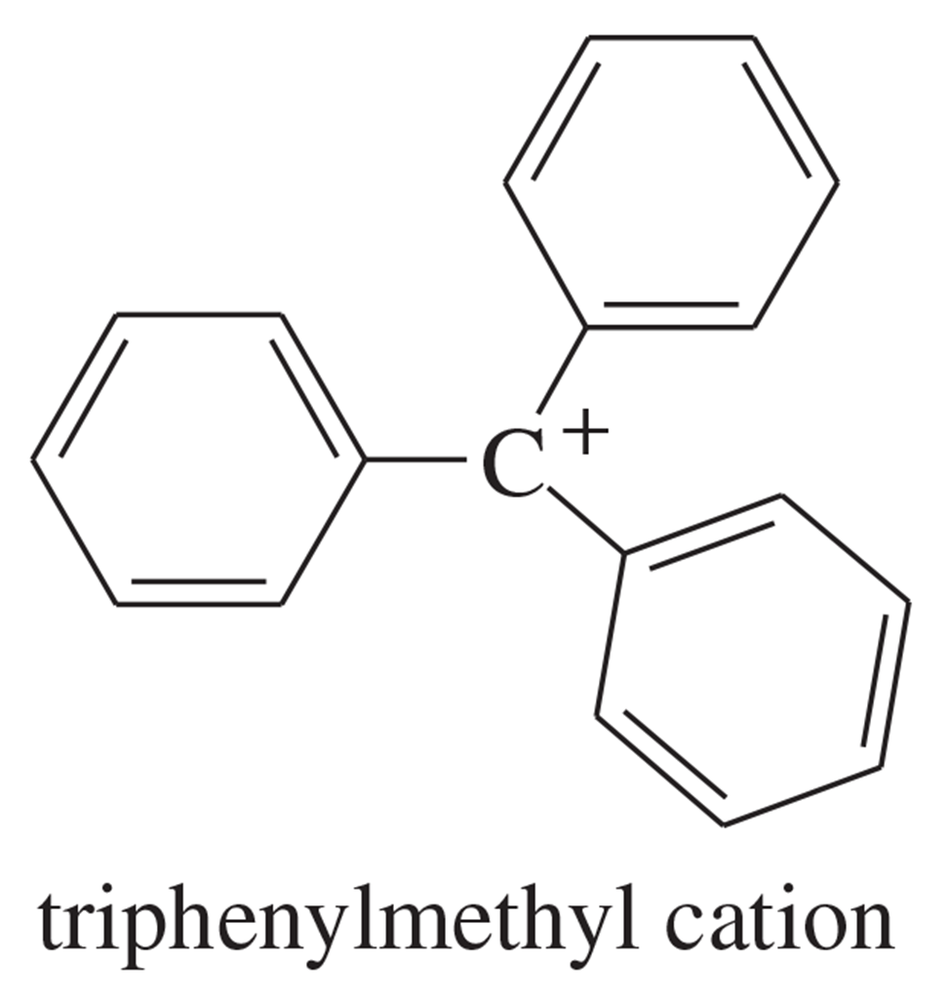
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 5:58m
5:58mMaster Determining Carbocation Stability with a bite sized video explanation from Johnny
Start learning