For each set of reactive intermediates, rank them in order of reactivity (1 = most reactive).
(a)
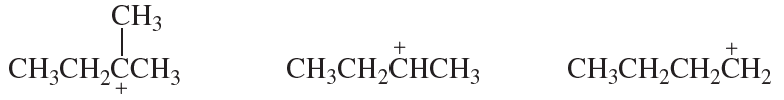
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 5:58m
5:58mMaster Determining Carbocation Stability with a bite sized video explanation from Johnny
Start learning