Textbook Question
Which is more stable: a methyl cation or an ethyl cation? Why?
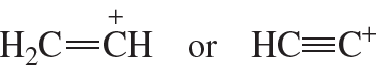
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 5:58m
5:58mMaster Determining Carbocation Stability with a bite sized video explanation from Johnny
Start learning