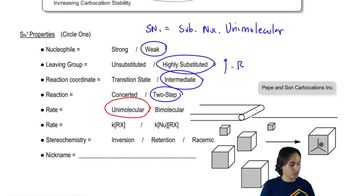
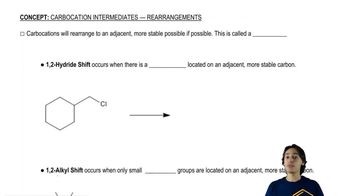
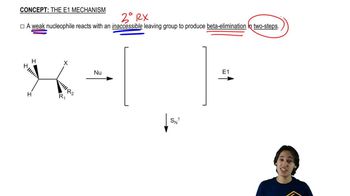
Deuterium (D) is the isotope of hydrogen of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly (5.0 kJ/mol, or 1.2 kcal/mol) stronger than the C―H bond. Reaction rates tend to be slower if a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step. This effect on the rate is called a kinetic isotope effect. (Review PROBLEM 4-57)
a. Propose a mechanism to explain each product in the following reaction.