Predict the products and mechanisms of the following reactions. When more than one product or mechanism is possible, explain which are most likely.
e. isobutyliodide + KOH in ethanol/water
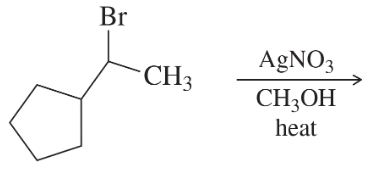
f. isobutylchloride + AgNO3 in ethanol/water
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: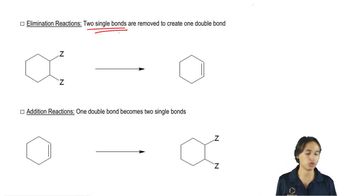



 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning