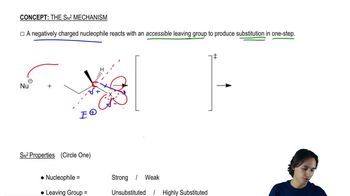
The solvolysis of 2-bromo-3-methylbutane potentially can give several products, including both E1 and products from both the unrearranged carbocation and the rearranged carbocation. Mechanisms 6-6 and 7-2 show the products from the rearranged carbocation. Summarize all the possible products, showing which carbocation they come from and whether they are the products of E1 or reactions.
<IMAGES>