Which of these reactions are likely to produce both elimination and substitution products?
a. 2-bromopentane + NaOCH3
b. 3-bromo-3-methylpentane + NaOMe. (Me = methyl, CH3)
c. 2-bromo-3-ethylpentane + NaOH
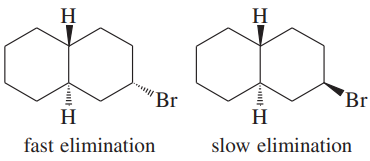
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:27m
2:27mMaster Overview of the flowchart. with a bite sized video explanation from Johnny
Start learning