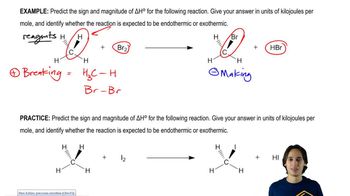
a. Propose a mechanism for the following reaction:
b. Use the bond-dissociation enthalpies given in Table 4-2 (page 167) to calculate the value of ΔH° for each step shown in your mechanism. (The BDE for CH2=CHCH2―Br is about 280 kJ/mol, or 67 kcal/mol.) Calculate the overall value of ΔH° for the reaction. Are these values consistent with a rapid free-radical chain reaction?