Reaction (c), on the other hand, is favored (∆G° < 0). Identify the bonds formed and broken and explain this result in light of (a) and (b).
(c)

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: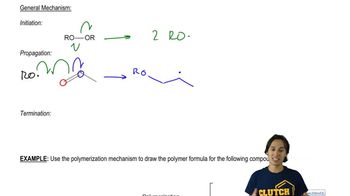
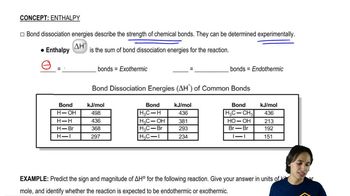
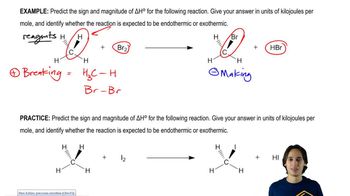

 4:09m
4:09mMaster How to calculate enthalpy using bond dissociation energies. with a bite sized video explanation from Johnny
Start learning