b. Draw the resonance forms for ozone (bonded O–O–O)
c. Sulfur dioxide has one more resonance form than ozone. Explain why this structure is not possible for ozone.
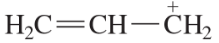
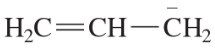
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:34m
3:34mMaster The rules you need for resonance: with a bite sized video explanation from Johnny
Start learning