How many valence shell electrons do each of the following elements contain? How many new bonds can each form?
(b) N
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: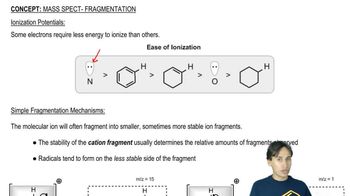


 2:14m
2:14mMaster What is a valence electron? with a bite sized video explanation from Johnny
Start learning