a. Find potassium (K) in the periodic table and predict how many valence electrons it has.
b. What orbital does the unpaired electron occupy?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: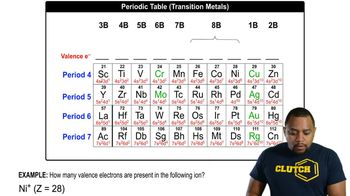


 2:14m
2:14mMaster What is a valence electron? with a bite sized video explanation from Johnny
Start learning