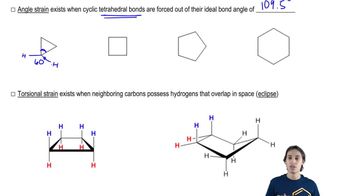
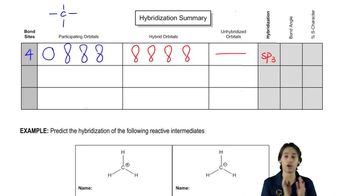
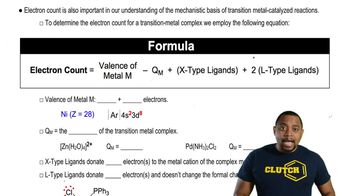
For each of the following line-angle drawings,
(i) give the number of carbons,
(ii) label the carbons,
(iii) tell how many hydrogens are on each carbon, and
(iv) draw the hybrid structural formula.
(c)
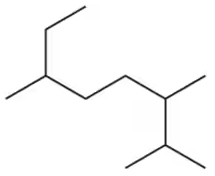
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: