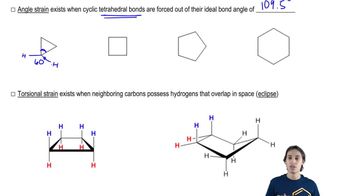
Given the line-angle drawings shown, answer the following questions:
(i) How many carbons are in each molecule?
(ii) How many hydrogens are at the circled carbon?
(iii) Is the indicated (→) carbon or 1° , 2°, 3°, or 4°?
(f)
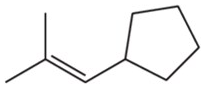
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: