Sodium amide (NaNH2) dissociates to give a sodium cation (Na+) and amide ion (NH2-) a very strong base. In the following three equations, identify which definition of base is being exemplified.
(c)
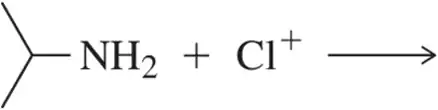
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:49m
2:49mMaster The Lewis definition of acids and bases. with a bite sized video explanation from Johnny
Start learning