For the following acid–base pairs, (i) complete the reaction; (ii) identify the acid (A), base (B), conjugate acid (CA), and conjugate base (CB);
(f)
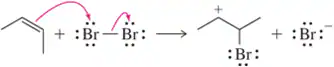
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:49m
2:49mMaster The Lewis definition of acids and bases. with a bite sized video explanation from Johnny
Start learning