Rank the given solvents in decreasing order of their ability to dissolve each compound.
Solutes:
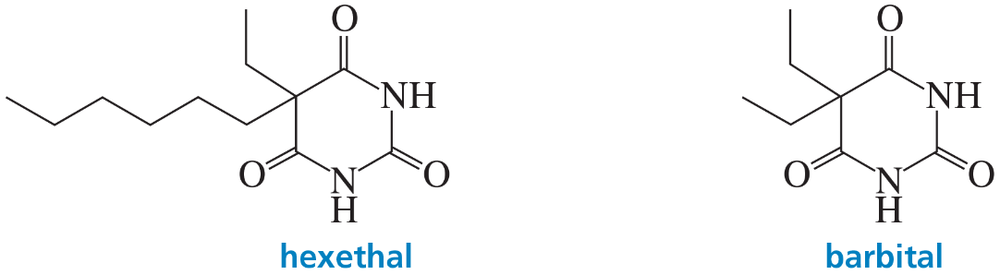
(a) NaOAc
(b)
(c)
Solvents:
ethyl ether
water
ethanol
dichloromethane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:47m
1:47mMaster Understanding “like dissolves like”. with a bite sized video explanation from Johnny
Start learning