The following reaction steps are shown using conventional electron pushing. (a) Draw the second product whose formation would have been rationalized with this same arrow.
(a)

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: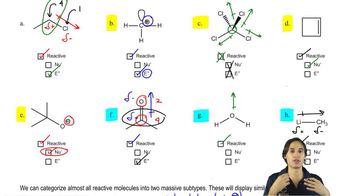


 5:14m
5:14mMaster How to tell if a molecule will be reactive or not. with a bite sized video explanation from Johnny
Start learning