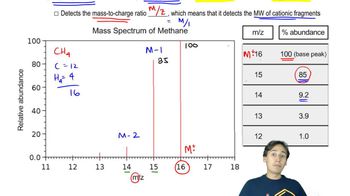
Primary alcohols have a strong peak at m/z = 31.
a. What fragment is responsible for this peak?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:28m
4:28mMaster Ionization Potentials with a bite sized video explanation from Johnny
Start learning