Choose the conformation in each pair that is most stable. If both are equally stable, then write 'no difference.'
(a)
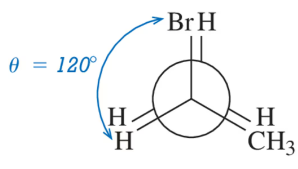
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: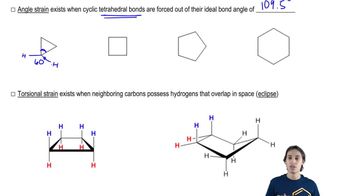
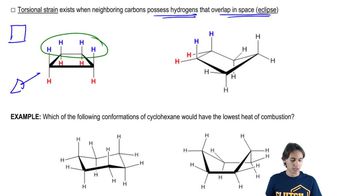

 3:11m
3:11mMaster How sigma bond rotation is visualized with a bite sized video explanation from Johnny
Start learning