Textbook Question
Although 2-methyl-1,2-propanediol is an unsymmetrical vicinal diol, only one product is obtained when it is dehydrated in an acidic solution.
b. Why is only one product obtained?
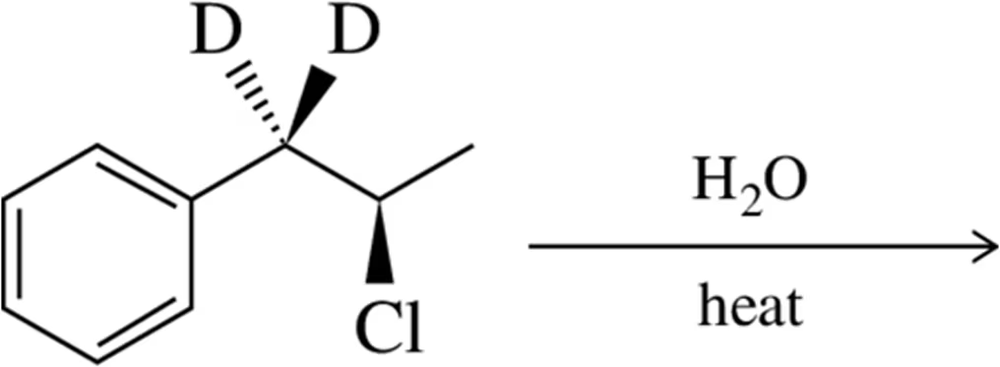
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 8:09m
8:09mMaster Drawing the E1 Mechanism. with a bite sized video explanation from Johnny
Start learning