Textbook Question
Predict the product of the following rearrangement-prone E1 eliminations.
(a)
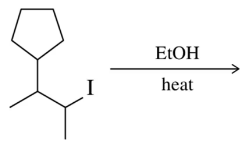
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: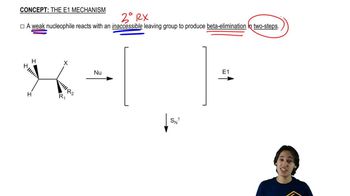
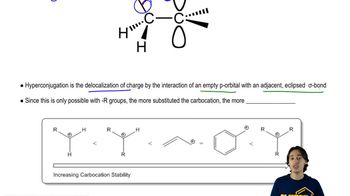

 8:09m
8:09mMaster Drawing the E1 Mechanism. with a bite sized video explanation from Johnny
Start learning