Predict the products formed when m-cresol (m-methylphenol) reacts with
(c) bromine in CCl4 in the dark
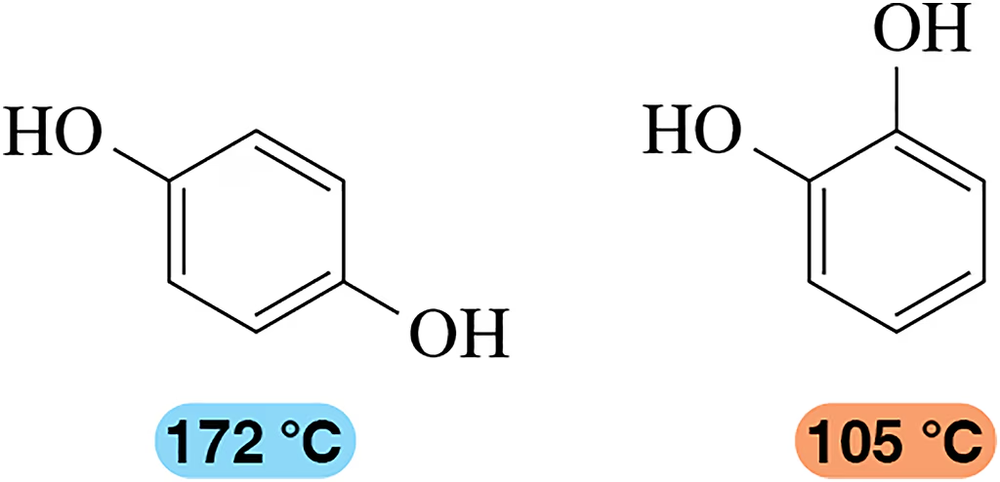
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:37m
2:37mMaster Donating vs Withdrawing Groups with a bite sized video explanation from Johnny
Start learning