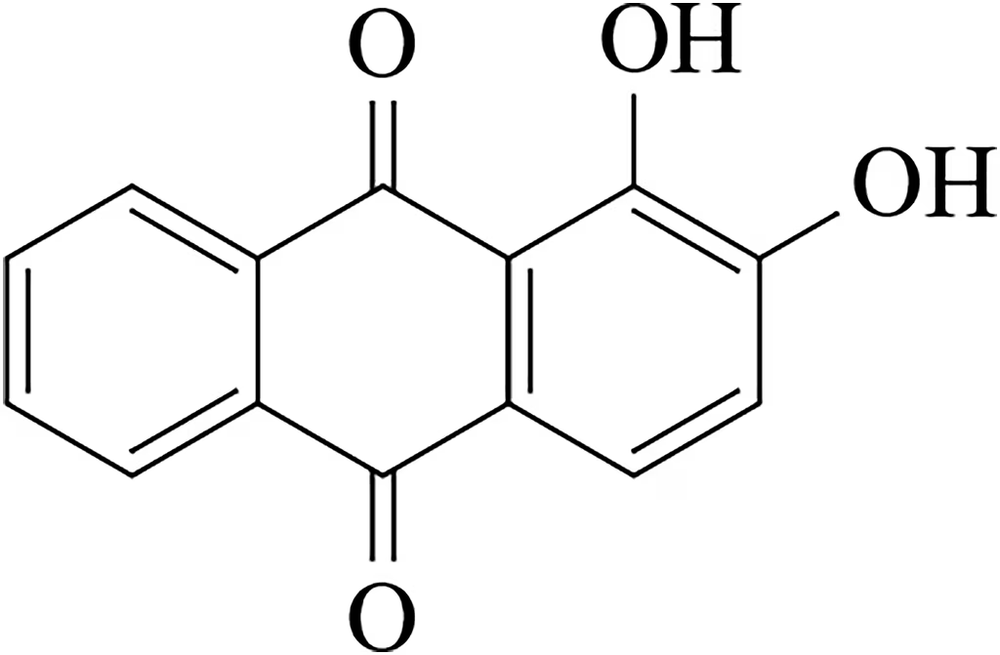
The following compounds are only slightly soluble in water, but one of them is very soluble in a dilute aqueous solution of sodium hydroxide. The other is still only slightly soluble.
(a) Explain the difference in solubility of these compounds in dilute sodium hydroxide.