Multiple Choice
Which of the following groups in electrophilic aromatic substitution (EAS) reactions exhibit synergistic activation when present on a benzene ring?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
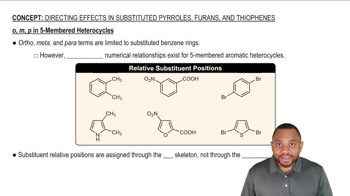


 8:48m
8:48mMaster EAS with Polysubstituted Benzene with a bite sized video explanation from Johnny
Start learning