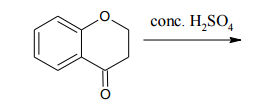
For each of the following compounds, indicate the ring carbon(s) that is/are nitrated when the compound is treated with HNO3/H2SO4:
a.
b.



 Verified step by step guidance
Verified step by step guidance
 8:48m
8:48mMaster EAS with Polysubstituted Benzene with a bite sized video explanation from Johnny
Start learning
