For each of the following compounds and ions,
1. Draw a Lewis structure.
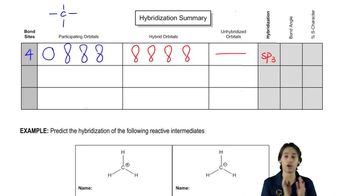
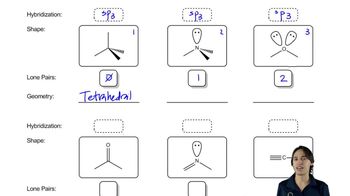
2. Show the kinds of orbitals that overlap to form each bond.
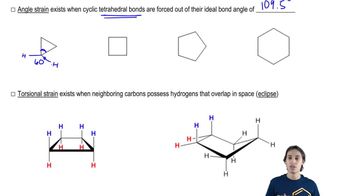
3. Give approximate bond angles around each atom except hydrogen.
c. CH2=N–CH3
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: