What is the hybridization of all the atoms (other than hydrogen) in each of the following?
What are the bond angles around each atom?
c. -CH3
d. ⋅CH3
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: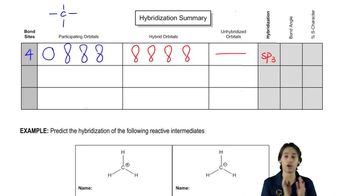


 7:44m
7:44mMaster Molecular Geometry Explained. with a bite sized video explanation from Johnny
Start learning