Three arene oxides can be obtained from phenanthrene.
d. Which of the three phenanthrene oxides is most likely to be carcinogenic?
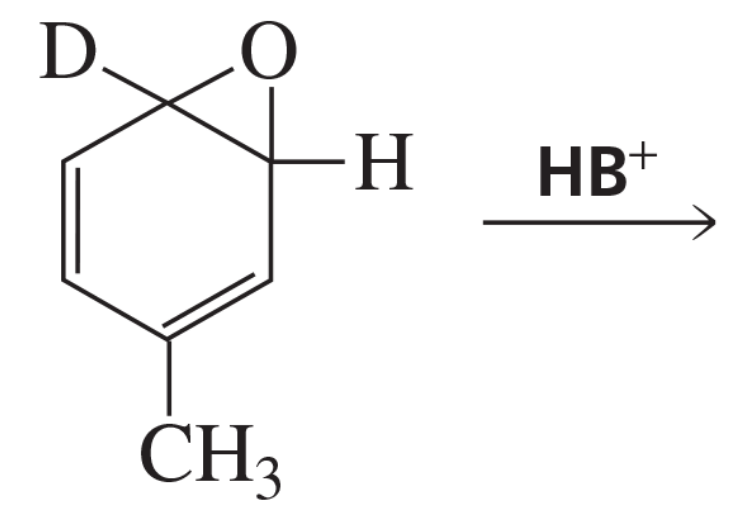
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 4:34m
4:34mMaster Acid-Catalyzed Epoxide Ring-Opening with a bite sized video explanation from Johnny
Start learning