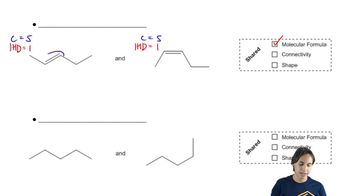
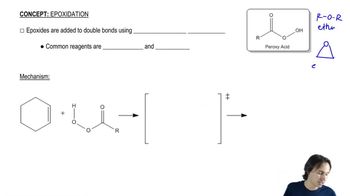
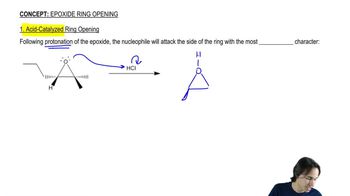
The existence of the NIH shift was established by determining the major product obtained from rearrangement of the following arene oxide, in which a hydrogen has been replaced by a deuterium.
b. What would be the major product if the carbocation forms phenol by losing H+ or D+, rather than by going through the NIH shift?