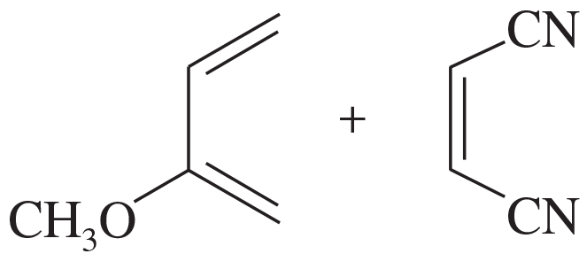
Predict the product of the following reactions. [When all of the reactions from this chapter are shown together, you must first decide which type of reaction each is. Is it a Diels–Alder, an electrocyclic, or a sigmatropic rearrangement? Drawing the product will be easier once this determination is made.]
(a)