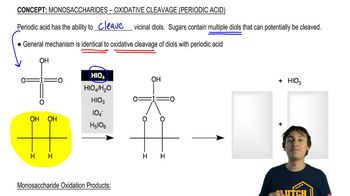
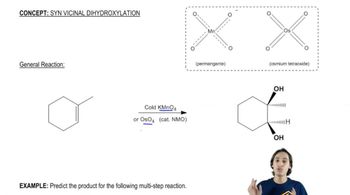
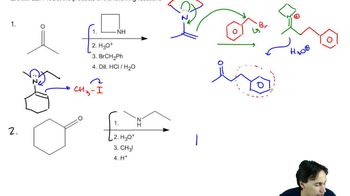
Predict the products of the reactions of the following compounds with:
1. chromic acid or excess sodium hypochlorite with acetic acid.
2. PCC or NaOCl (1 equivalent) with TEMPO.
a. cyclohexanol
b. 1-methylcyclohexanol
c. cyclopentylmethanol
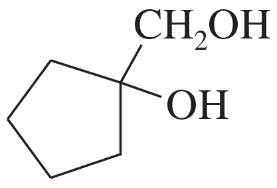
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: