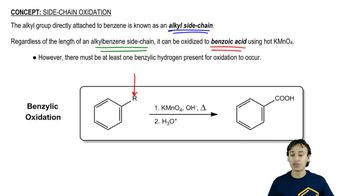
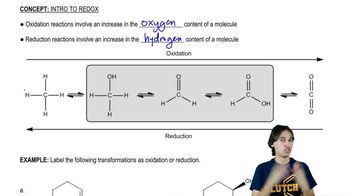
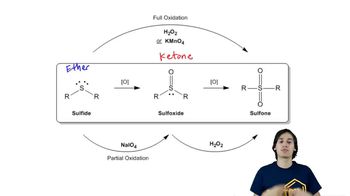
Give the structure of the principal product(s) when each of the following alcohols reacts with (1) Na2Cr2O7/H2SO4, (2) PCC, (3) DMP, and (4) 1 equiv NaOCl-TEMPO.
a. octan-1-ol
b. octan-3-ol
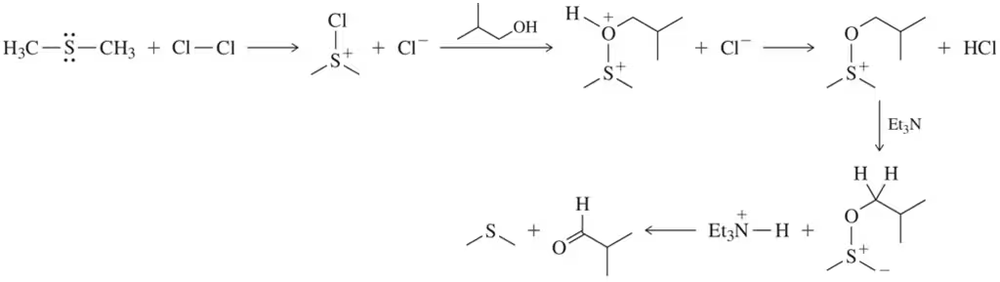
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: