Construct a graph, similar to Figure 3-11, of the torsional energy of 3-methylpentane along the C2―C3 bond.
c. Begin your graph at the 0° dihedral angle and begin to turn the front carbon.
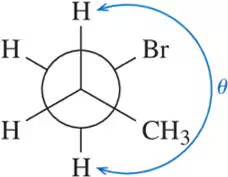
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:11m
3:11mMaster How sigma bond rotation is visualized with a bite sized video explanation from Johnny
Start learning