Identify the location and type of charge on the hexapeptide Lys-Ser-Asp-Cys-His-Tyr at each of the following pH values:
c. pH=7
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: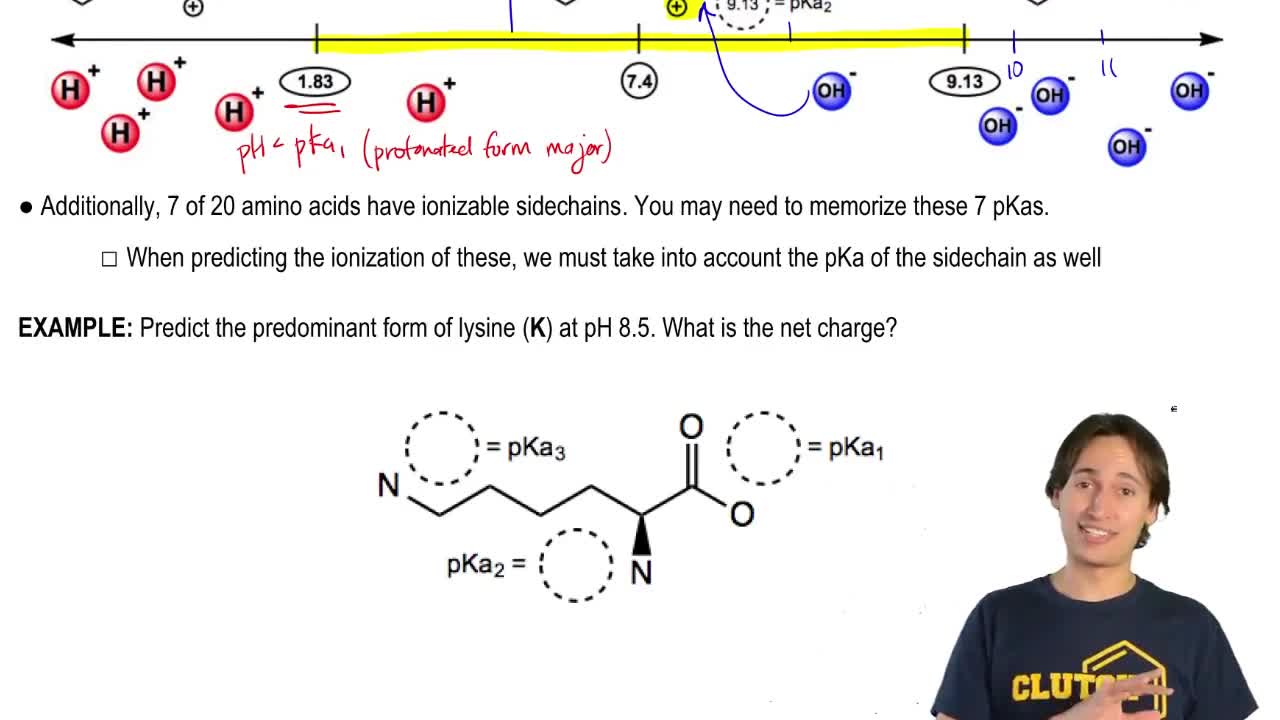



 4:41m
4:41mMaster Why Amino Acids Exist as Zwitterions with a bite sized video explanation from Johnny
Start learning